Methanol (Methyl Alcohol)
Hosea Chem® has been supplying Methanol (CAS 67-56-1) with high quality and competitive price for many years, covering most of the European, American, etc. Send Inquiry
Product Description
Methanol
Chemical Name:Methanol;Methyl Alcohol;CAS 67-56-1
EINECS No.: 200-659-6
Chemical Formula: CH4O
Molecular Weight: 32.04
Melting point: -98°C
Boiling point: 64.5-65.4℃
Flash point: 12°C
Density at 25°C: 0.791 g/mL
Molecular Structure:
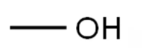
Description
Methanol is the simplest fatty alcohol. It is a colorless, flammable, irritating liquid with a boiling point of 64.7°C, a melting point of -93.90°C, and a relative density of 0.7913. Soluble in water and most organic solvents. Its severe toxicity can damage the optic nerve. Once swallowed, it can make the eyes blind and even cause death. Methanol has the general properties of a primary aliphatic alcohol. The three hydrogen atoms on a carbon atom with a hydroxyl group can be oxidized, orderly generating formaldehyde, formic acid, and carbon dioxide. Therefore, it is largely used in the synthesis of formaldehyde. Methanol is easily converted into important organic synthesis intermediates such as methyl carboxylate, methyl chloride and methylamine, and it is also applied as important organic solvents, extraction agents and alcohol denaturants.
Methyl Alcohol Standard
Appearance: Clear Colorless Liquid Free From Particulates
Content %≥: 99.0
Color <(APHA): 10
Density at 25°C: 0.791 g/mL
Vapor pressure (50℃): 410 mmHg
Refractive index n20/D: 1.329
Explosive limit %(v): 5.5-44
Relative Polarity: 0.762
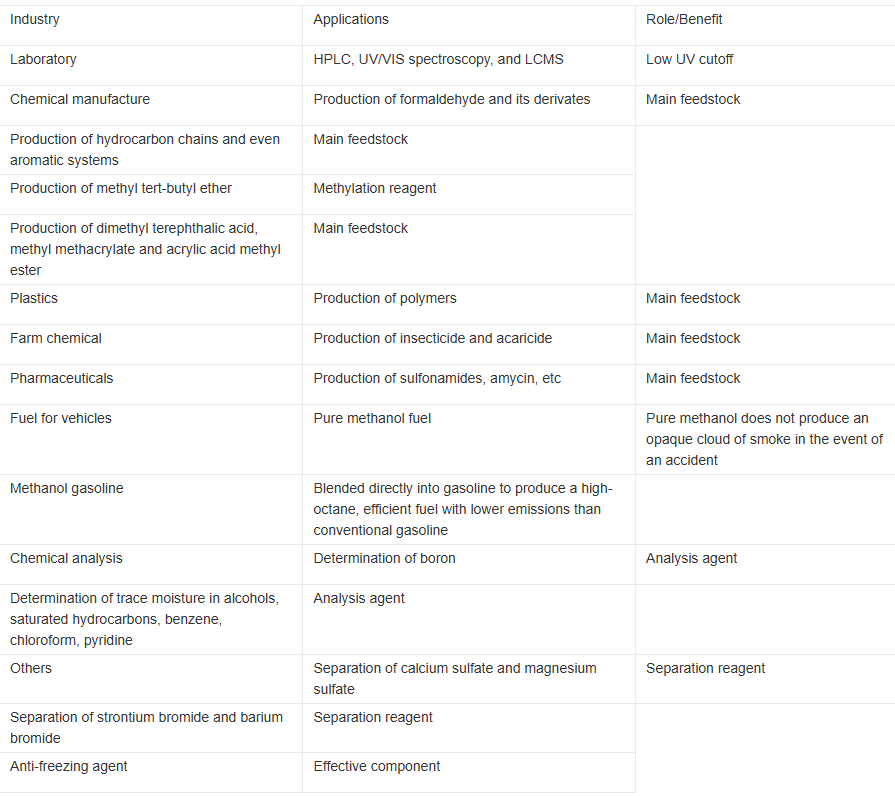
Application
Methanol is an important chemical raw material for fine chemicals. Its carbonylation at 3.5 MPa and 180-200° C in the presence of catalyst can produce acetic acid and further produce acetic anhydride. It reacts with syngas to prepare vinyl acetate in the presence of catalyst; reacts with isobutylene to produce tert-butyl methyl ether; prepare dimethyl oxalate through oxidization and carbonylation, and a further hydrogenation to produce ethylene glycol; reacts with toluene under catalyst and simultaneous oxidization to produce phenylethyl alcohol. It can be used as a good solvent, as a pesticide raw material, as an antifreeze agent, as a fuel and fuel additive (this is receiving increasing attention in environmental protection field). It is the main raw material in the preparation of formaldehyde, the raw material in medicine and spices production, a solvent in dyes and paint industries, the raw material in preparation of methanol single cell protein and synthesis of methyl ester.
Storge & Handling
Methanol should be used only in areas free of ignition sources, and quantities greater than 1 liter should be stored in tightly sealed metal containers in areas separate from oxidizers.
Packing
200KG/Drum










